Abstract
Introduction:
Hydroxyurea (HU) was the first FDA-approved therapy for sickle cell anemia (SCA) and remains the most well-established disease-modifying therapy, reducing painful crisis and improving morbidity and mortality. HU improves outcomes through the upregulation of fetal hemoglobin (HbF). Dosing is highly variable with doses ranging from <10-35 mg/kg/day. Most dosing strategies escalate with a goal of mild myelosuppression, commonly defined using an absolute neutrophil count (ANC) between 2,000-4,000 and platelets >80,000. Macrocytosis is often used as a surrogate marker for compliance and effect. Despite its well-established effectiveness, clinical use remains limited with only 12% of adults with SCA taking HU. The reasons for this are multifactorial but include skepticism by both providers and patients regarding its effectiveness in the adult population. Some experts suggest up to 30% of adults are non-responders with no significant HbF change, and many believe that the effect of HU fades with time. Dosing strategies are usually quite conservative to prevent excessive myelosuppression, though dose is highly correlated with clinical effect and ultimate %HbF. There is very limited real-world data regarding long term effectiveness of HU in adults with SCA over time.
Methods:
We retrospectively analyzed the records of the 109 adults with SCA currently treated in our multidisciplinary sickle cell clinic. Data from 1/1/2011-6/30/2021 was collected, including genotype, HU prescription status, current and maximum laboratory values (HbF, MCV, ANC), and number of admissions for painful crisis.
We performed Wilcoxon rank sum testing between pts prescribed and not prescribed HU and measures of HbF (peak, average and current) and number of admissions for painful crisis over the study period.
Results:
Among 106 pts (3 excluded from analysis, 2 for lack of data and 1 for post-transplant status), 79 are currently prescribed HU (75%). Among our 63 pts with HbSS genotype 58 are prescribed HU (92%). Average HbF over time for all pts prescribed HU was 11.9%. Average peak HbF was 18.1% and average current HbF is 12.4%. In the pts not prescribed HU HbF average, peak and current levels were 5.5%, 6.7%, and 5.0% respectively.
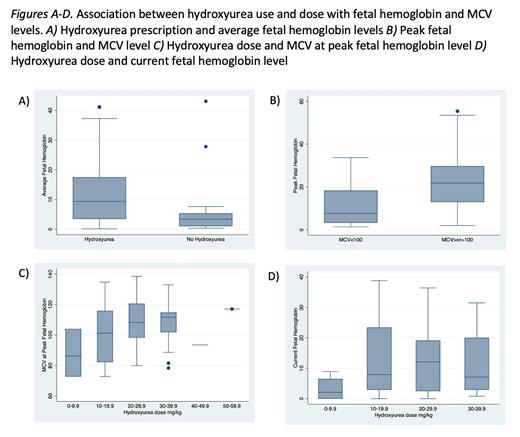
HU prescription was significantly associated with increased HbF at peak, average, and current levels (p-value <0.001, Figure A). HU prescription was also significantly associated with decreased number of admissions for painful crisis (p-value 0.001). Among pts prescribed HU, MCV levels >100 at time of peak HbF were associated with higher peak levels than pts with MCV <100 (p-value <0.001, Figure B). Larger peak HU doses were also correlated with higher MCVs at the time of peak HbF levels (Figure C). Larger current HU doses were also significantly associated with higher current HbF levels (Figure, D). Doses >9.9m/kg were associated with significantly higher HbF levels however there was no significant difference between dose levels 10-19.9mg/kg and 20-29.9 or 20-29.9 and 30-39.9mg/kg (p-values 0.02, 0.68 and 0.84 respectively).
Among pts prescribed HU, 34 obtained ANC levels <4000 at the times of peak HbF and 24 at the current dosing level (43% and 30% respectively). 24 pts obtained both MCV>100 and ANC <4000 at time of peak HbF and only 11 achieved both at current dosing levels (30% and 14% respectively).
Conclusion:
In our adult multidisciplinary sickle cell clinic prescribing rates are well above current HU usage for adults with SCA. Our data notes that elevated HbF levels can be maintained over long periods of time. HU prescription was significantly associated with increased HbF levels and reduced admissions for painful crisis. This was despite a majority of pts not meeting target prescribing levels of ANC <4000 and MCV >100. While higher peak HbF levels were significantly associated with higher HU doses, this was only true for doses above 9.9 mg/kg. Further investigation is needed to explore the factors contributing to suboptimal HbF and MCV response, including careful assessment with medication adherence and dose selection. Overall, these data illustrate the importance of dosing and suggest that one size dosing of HU for adults with SCA does not fit all. We hypothesize that there may be a role individualized dosing strategies in adults with SCA, incorporating factors such as pharmacokinetics and renal function to achieve the optimal dose for each patient.
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal